Tiny bubbles
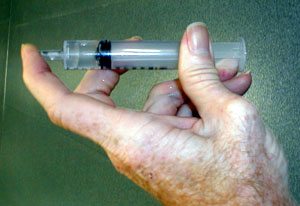
A partial vacuum can be generated by covering the end of a syringe of
water,
then pulling on the plunger.
Tiny bubbles

A partial vacuum can be generated by covering the end of a syringe of
water,
then pulling on the plunger.
Introduction
Water will boil at room temperature if its pressure is lowered.
Material
To Do and Notice
Fill a syringe a quarter full of water. Try to fill it so that there is a little air in the syringe as possible.
Cover the filler hole with a finger.
Pull on the plunger. (See the first image above)
Notice that as you pull on the plunger it pulls back on you. The pressure inside the syringe is reduced below atmospheric pressure so there is a net force exerted by the outside air pushing the plunger back in to the syringe and the gas inside the syringe pushing outward less strongly.
Notice also that a space appears within the plunger that is not filled by water.
Allow the plunger to slide slowly back into the syringe. Notice if there is an air bubble in the syringe.
Pull the plunger out again.
Release the plunger suddenly.
Notice that it snaps back quickly.
Pull on the plunger a second time. Notice that
this time bubbles form in the water.
The water appears to be boiling.
Repeat the experiment with carbonated water.
What’s Going On?
When you pull on the plunger you increase the volume inside the syringe and decrease the pressure on the water. A space appears above the water. In this space there is a partial vacuum. It is not a perfect vacuum because it has some water vapor in it as well as some air.
Tap water has air dissolved in it. When you reduce the pressure the dissolved air comes out of solution forming bubbles. When you slowly allow the plunger to slide back in to the syringe the air that has come out of solution stays out of solution. Any bubble that forms and does not go away after pulling out the plunger and then allowing it to return is a bubble made of air which came out of solution.
Water vapor goes from a gas to a liquid very quickly. Any gas bubbles that form when the syringe is pulled out and then go away when the syringe is allowed to return were full of low pressure water vapor. When these bubbles form inside the liquid we say that the liquid boils.
It is difficult for small bubbles to form to allow boiling to start within a clean liquid. However when you pull out the plunger and allow it to snap back you create small "seed" bubbles throughout the water. the next time the pressure is reduced boiling happens at these seed bubbles.
You can understand how hard it is to make a small bubble by remembering the last time you tried to blow up a balloon. The balloon is really difficult to get started, but once it is started it is much easier to inflate. The same rule applies to bubbles in water.
Optional experiment with carbonated water.
Carbonated water has carbon dioxide dissolved in it. The carbon dioxide in the bottle is under pressure. When you release the pressure by opening the bottle the carbon dioxide comes out of solution forming bubbles. Often the bubbles rise in long vertical lines from a seed bubble in a scratch or pit in the wall or bottom of the glass. When you pull on the syringe and decrease the pressure below atmospheric pressure the carbon dioxide comes out of solution even more rapidly. Bubbles form in the water. By tapping the sides of the syringe you can force all of the bubbles together into one larger bubble. When you release the plunger and allow the pressure to return to atmospheric pressure the carbon dioxide bubble does not all go away. Some, but not all, of the carbon dioxide will slowly re-dissolve in the water.
So What?
The difficulty of starting a bubble explains why it is dangerous to boil water in a microwave oven. If you are heating water in a clean ceramic or glass cup it is possible that water can be heated above the boiling point and yet be unable to form bubbles. the water is said to be superheated. When you remove the water from the microwave you can jiggle it and shake loose bubble "seeds" so that the water suddenly erupts into boiling, splattering hot water around.
Etc.
The water in the syringe is actually boiling at room temperature. If you reduce the pressure even further using a vacuum pump it is possible to have the water boil at the freezing point. So that you can have liquid water, solid ice and bubbles of gas together forever. This is called the triple point of water, where all three phases exist in equilibrium. The triple point of water is 0 °C and 6 milliners. (a Bar is one atmosphere of pressure.) The triple point of water is found at places on the surface of Mars.
Reference: The exhibit "Water Freezer" at the Exploratorium reduces the pressure on a sample of water until it boils. The water continues to boil until it freezes.
|
Scientific Explorations with Paul Doherty |
|
25 June 99 |