Paleotemperature
Oxygen isotopes in antarctic ice and sediment cores record the temperature of the ocean in ancient times.
A teacher Institute workshop on 14 December 2008
To see these activities as taught by Paul Doherty search the Exploratorium Webcasts for "Antarctic Ice Hands-On Demo with Paul Doherty"
Ice Cores
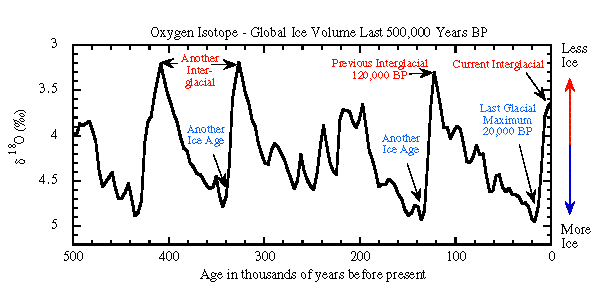
Water molecules have one of the best known chemical compositions, H2O. Water liquid in the oceans evaporates to become water vapor in the atmosphere. The water vapor in the atmosphere condenses into water droplets that make clouds rain and snow. The snow falls on Antarctica where it accumulates year after year. Scientists drill ice cores into that antarctic ice. They can count the yearly layers in the ice and determine the age of the ice, just as you count the yearly rings in a tree to determine its age. The ice contains bubbles of air from the past which can be analyzed to discover the ancient composition of the atmosphere. The oxygen isotopes in the water of the ice itself also contain a record of the temperature of the surface of the ocean that provided the water molecules in the ice. The antarctic ice preserves a record of the earths temperature for the last 600,000 years.
Sediment Cores
Scientists also drill cores in oceanic sediments. The shells of organisms in these cores are made of calcium carbonate, CaCO3 the oxygen isotopes in these shells also record the temperature of the ocean surface. The sediment record stretches back millions of years.

Notice in this ice core record that more ice means more oxygen 18 in the oceans.
Water Vapor, H2O makes up a variable amount of the earths atmosphere, up to 4 percent in hot, humid conditions.
What is the Atmosphere made of? DRY air is 78% N2, 21% O2, and 1% Ar
However, real air has a variable amount of H2O up to 4%
It also contains trace gasses such as carbon dioxide, and methane.Atmosphere Composition Model Model the composition of the atmosphere using dyed grains of rice.
Stephanie Chasteen olds a 1 liter container with dyed rice grains showing the composition of the atmosphere.
Isotopes
Try the modeling isotopes activity.
The nucleus of every atom has protons and neutrons. The number of protons determines the chemical identity of the element. A nucleus with one proton is a hydrogen nucleus. Nuclei may also have different numbers of neutrons. Hydrogen is found with no neutrons, one neutron which is called dueterium H2, and two neutrons called tritium H3.
Two nuclei each with the same number of protons and different numbers of neutrons are called isotopes.
Dueterium is a stable isotope of hydrogen it never radioactively decays.
Tritium is a radioactive isotope of hydrogen.
The most common stable isotope of oxygen is oxygen-16, 16O. This isotope accounts for 99.76% of all the oxygen on earth.
The second most common isotope is oxygen-18, 18O, Which is 0.2% of the oxygen on earth. Oxygen 18 is 2 parts per thousand of the total oxygen.
The third stable isotope of oxygen is Oxygen-17,17Obut this makes up only 0.04% of the oxygen on earth.
Oxygen-18 is 12% heavier than oxygen-16.
The amount of an isotope contained in a sample is measured by a mass spectrometer. The amount is usually reported as a ratio between the two ratios: oxygen-18/oxygen-16 in a sample and the ratio oxygen-18/oxygen-16 in a standard reference type of water. The standard reference is called the Standard Mean Ocean Water, SMOW.
It is reported as the ratio of ratios! It is reported in parts per thousand often called parts per mil. (Caution this is not parts per million!)
d 18O = (18O/16Osample) / (18O/16OSMOW)
Isotope Evaporation, use heavy and light balls in a shallow box to model the evaporation of water molecules with heavy and light isotopes of oxygen.
|
Scientific Explorations with Paul Doherty |
|
22 November 2008 |