
Modesto gathers the materials for an aluminum air battery.
Foiled again
Introduction
A simple battery can be made from aluminum foil, salt water and activated charcoal that will make 1 volt and 100 milliamps.
Material

Assembly
Make a saturated salt solution by mixing salt and water in the bowl. Some undissolved salt should remain in the bottom of the bowl after mixing.

Shaking salt all around the vicinity of the bowl Modesto Tamez
shows the chemical precision mixing needed to make the salt water
solution...none.
Lay down a sheet of aluminum foil (approximately 30 cm long by 15 cm wide.)
Cover the aluminum foil with a doubled over piece
of paper towel.
Soak the paper towel in the salt solution before using it to cover
the aluminum.

Chef Modesto begins to prepare his burrito battery. He places salt
water soaked paper towel over a sheet of aluminum foil.
Pour a layer of activated charcoal over the wet paper. Make it about a centimeter thick.

Make a layer of activated charcoal over the wet paper.
Place one metal lead on top of the carbon.
Clip the second lead to the aluminum foil.
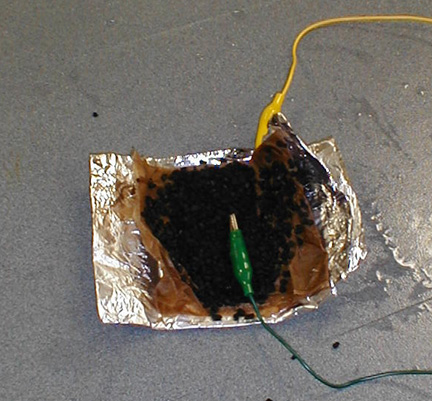
Fold the aluminum foil over to make a burrito.
Important points:

The aluminum foil is folded over to make a burrito with one
electrical lead in its center.
Attach a "flag" made of masking tape to the shaft of the motor.
Clip the other electrical lead to the aluminum foil.
Clip both leads to the motor.
To Do and Notice
Notice that nothing happens.
Push down on the burrito and notice that the motor spins rapidly.

Pressing on the battery increases the current it delivers to the
motor.
Measure the voltage and current produced by the battery, perhaps 1 volt and 100 ma. Notice that the outside aluminum electrode is the low voltage electrode, it is the source of electrons.
What's Going On?
This is called an aluminum air battery. The reaction that powers the battery occurs between the aluminum foil and oxygen from the air. The battery will deliver power for tens of minutes as the aluminum oxidizes.
Activated charcoal has many gas pockets giving it a large surface area this surface area provides the oxygen electrode.
The reaction with aluminum occurs in aqueous solution.
The maximum current delivered by the battery is determined by the voltage produced by the battery and by the internal resistance of the battery. The internal resistance can be reduced by making the electrode areas larger. It is also reduced by using salt water which has a lower resistance than pure water. The charcoal is a conductor, but its resistance decreases as the grains are pressed together.
So What?
The aluminum air battery produces useful amounts of power from safe chemicals.
Acknowledgments:
This activity was developed by Modesto Tamez from materials presented at the Exploratorium by Japanese teachers from Galileo Workshop.
|
Scientific Explorations with Paul Doherty |
|
10 July 2000 |