A rubber band stretches out to the first excited
state.

A rubber band stretches out to the first excited
state.
Introduction
Use a paper plate and rubber band to model the energy level changes that lead atoms to emit light.
Material
Assembly
Use the pen to poke a small hole through the center of the plate. (If you balance the plate on one finger, the finger will be under the center.)
Tie the rubber band to the middle of the toothpick.
Push the rubber band through the hole in the center of the plate so that the toothpick is left on the bottom of the plate.
Use the pen to draw a circle on the plate with a radius equal to the unstretched length of the rubber band.
Draw a circle around the rim of the plate.
Draw a circle half way between the two circles you just drew.
To Do and Notice.
Pull the rubber band from the inner circle to the
middle circle.
Feel the work you must do to stretch the rubber band.
Pull the rubber band from the middle circle to the
rim.
Feel that you must do more work.
Holding the band at the rim with one hand, place a finger in the middle of the band touching the middle circle. release the rubber band. Notice that it snaps in and stops at your finger. Feel the energy it delivers to your finger.
Now put your finger in the center of the rubber band touching the inner circle and release it. Feel the energy release.
Pull the rubber band to the outer edge once again. Put your finger in the center of the band touching the inner circle and release the rubber band. Notice that it snaps into your finger delivering more energy than before.
What's Going On?
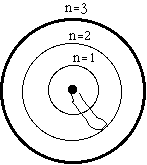
The circles model the energy levels of an atom.
The rubber band models the potential energy of an
electron.
The innermost circle is the lowest energy level, the "ground state," it is assigned quantum number n=1.
The middle circle is assigned quantum number n=2, and is the first excited state. You have to add energy to the rubber band to stretch it out to state number 2.
The outer circle is the second excited state with n=3. You have to add even more energy to move the rubber band end out to state number 3.
This rubber band system models the energy levels of atoms.
In atoms, electrons move between energy levels.
When they move out from an inner, low energy level, they must be
given energy.
Usually the energy is added by a collision with another atom, or by absorption of a photon of light.
When the electrons drop into lower energy levels they release energy, usually by emitting a photon. But sometimes during a collision with another atom.
Higher energy changes, from the third level to the first release higher energy photons, e.g. blue or UV light.
Lower energy changes, from the third to the second energy level, release lower energy light e.g. red light.
Etc.
In a real atom the force gets weaker as the
distance increases,
while with this rubber band model the force gets stronger.
|
Scientific Explorations with Paul Doherty |
|
11 November 2002 |