Measuring the voltage across a battery.
An activity with potential
Introduction
Voltmeters measure the voltage difference between two points touched by their leads.
Material:
A commercial voltmeter
a 1.5 volt cell such as a AA, C or D cell.
Optional, any solar cell
To Do and Notice
Measure the voltage across one, “1.5 volt
battery”.
Details
What voltage does the battery claim to be? (1.5 volts)
Set the meter scale to measure direct current DC voltage use the
first scale larger than 1.5 volts. Perhaps 2, 5, 10 or 20
volts.
Hook up a bulb to a battery, measure the voltage across the bulb by touching the two leads of the meter to the two leads of the bulb.

Voltage
Your answer is probably not 1.5 Volts but it
should be close. The voltage across older batteries will be
lower.
If it isn’t see “troubleshooting” below.
Measure the voltage produced by the solar cell. How does the voltage change when the solar cell is illuminated by different amounts of light? In general you will measure slightly higher voltages when brighter light shines on the solar cell.
Measure the voltage across combinations of 2
batteries.
Each battery by itself will produce an increase in voltage of about
1.5 volts between its negative end and its positive end.
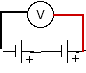
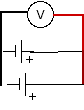
Try batteries: in series, with the positive end of one touching the negative end of the other; in opposition, with the positive end of one touching the positive end of another; and in parallel, with both positives and negatives joined.
Optional. Measure other cells such as C and D cells, notice that they produce the same voltage difference between their ends as do AA cells.
Troubleshooting
If the meter needle “goes the wrong way” then reverse the two meter leads, the red lead should go to the positive end of the cell&emdash;the end with the bump on it&emdash; while the black lead should go to the negative end. (And, of course, the black lead should go to the COM, or common, connection on the meter and the red lead to the V, or voltage, connection.
If your answer is not between 1.4 and 1.7 volts
then you are probably reading the wrong set numbers, ask a neighbor
for help.
If they too get an answer far from 1.5 volts then try another battery
Test the battery first on another meter to make sure it gives the
correct voltage. If the voltage is still way off try another
meter.
If your meter does not work swap connector leads with someone whose
meter does work. Were your leads bad or was it the meter?
What you have just done is called troubleshooting, in particular this
is troubleshooting by substitution, and is one of the most useful
skills an electronics technician can possess
What’s going on?
What is voltage
We know from electrostatics experiments that there are positive and negative electric charges. These charges come in units equal to the charge on the proton &emdash; Milliken discovered this by watching the charge on tiny oil drops. The charge on the drops was always an integer multiple of one basic charge which was equal in magnitude to the charge on an electron or proton. The SI unit of charge is the coulomb, one coulomb, C, contains 6.2 x 1018 proton charges. The coulomb is a collective term for charge just like a dozen or a gross are collective terms for eggs. So when I say “coulomb” picture 6 billion billion electric charges.
Like charges repel each other, so, to bring one positive charge next to another I have to do work. The work I do forcing the charges together is stored as potential energy, electrical potential energy. The electrical potential energy turns into kinetic energy if I release the charge and allow it to be repelled away. Electrical potential energy, like all energy, is measured in joules. (By the way, one joule is the energy needed to raise a quarter pounder hamburger patty with a mass of 0.1 kg through a height of 1 meter near the earth.)
The electrical potential energy per coulomb of
charge is called the potential. Yikes! what a confusing name.
Potential is the potential energy per charge. Another name for
potential is voltage, V. As an analogy, compare the difference
between the total price for 2 kg of hamburger and its price per kg.
The total price $5.24 corresponds to the total electrical potential
energy the price per kg corresponds to the potential or the voltage.
(By the way, the price per kg is $2.62.) If I move a coulomb of
positive electric charge to a place with a voltage that is higher by
one volt then I must do one joule of work on the charges. One volt is
one joule per coulomb. Another analogy for voltage is height. To
raise a 1 kg mass to a height of 3 meters requires 30 joules of work.
The mass then has 30 J of gravitational potential energy.
(For those of you who like equations,
U = mgh
potential energy, U, equals
mass , m, times
the acceleration of gravity, g,
times height change, h.)
The gravitational potential is the energy per kilogram which is
proportional to height. (U/m = gh). So in an electrical circuit you
can think of the change in height of a mass as an analogy for voltage
differences for charge.
The analogy continues. Just as balls with positive
mass tend to roll downhill, positive electric charges move from
places of high potential to places with lower potential. So positive
charges move under electrical forces from the positive terminal of
the battery through resistors to the negative terminal.
There are no negative masses, however, helium balloons in air behave
a lot like negative masses. Balloons tend to rise when gravity pulls
downward. So too, negative charges move from places with low voltage
to places with higher voltage. Thus, negative electrons move outside
of a battery from the negative or low voltage terminal to the
positive or high voltage terminal.
How is voltage created?
The 1.5 volt batteries you are measuring are
carbon-zinc cells. The metal case is lined with zinc which is
attached to the negative terminal, there is a carbon rod in the
center attached to the positive terminal. Between the carbon and the
zinc there is a black paste made from manganese dioxide, carbon, and
ammonium and zinc chlorides. In the cheapest batteries there is also
a cylindrical flour and paste separator. Cells marked “heavy
duty” have a larger amount of zinc chloride and a better
mechanical design, that is they don’t leak as easily. Alkaline
cells on the other hand have a powdered zinc negative rod surrounded
by a highly corrosive potassium hydroxide electrolyte surrounded in
turn by manganese dioxide and carbon as an outer positive
cathode.
You may cut open a carbon zinc cell with a hacksaw, but you must not
cut open an alkaline cell due to the corrosive nature of the
electrolyte. If you do cut open a carbon zinc battery, discard the
paste. You may replace the paste with salt water or a lemon and
re-use the carbon rod and the zinc metal in your own homemade
electric cells.
Inside the battery positive charges move in the paste toward the
positive electrode. They thus move against the electric force. The
positive charges are moved by a chemical potential inside the cell. A
battery converts chemical energy to electrical energy. In the height
analogy, outside work must be done on a ball to raise it to the top
of a hill. From the top of the hill the ball will roll back to the
bottom without help. The outside work provided by your muscles in the
case of the ball is like the chemical work done in the
battery.
Etc.
The word battery, as in gun battery, really means a line of many things. The 1.5 volt cells we are using are really single cells and so are not really batteries, however, most people call them batteries. Nine-volt carbon-zinc batteries are made out of a stack of 6, 1.5 volt cells and so they really are batteries. Cut them open and see!
You can also open 9 volt alkaline batteries, inside you will find six small alkaline batteries in series, each carefully contained in a metal case. Do not open these metal cases the insides are corrosive.
|
Scientific Explorations with Linda Shore and Paul Doherty |
|
19 July 2001 |