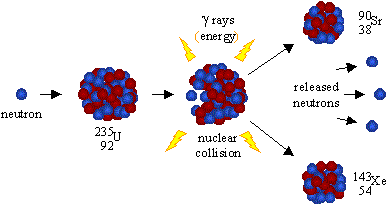
A neutron can trigger the fission of U235 into two roughly equal halves plus the emission of 2 to 3 neutrons.
Nuclear Fission
An isotope of element number 92, Uranium, U235, can spontaneously fission, that is, break into two roughly equal halves, releasing energy and two to three neutrons. Approximately 200 MeV are released in a nuclear fission. U235 has a very low spontaneous fission rate of 0.16 fissions per kg per second.
However, what is more interesting is that when a neutron strikes a U235 nucleus it can trigger nuclear fission and the release of 2 to 3 more neutrons that can then trigger fission in other nuclei.

A neutron can trigger the fission of U235 into two roughly equal halves plus the emission of 2 to 3 neutrons.
Some nuclei spontaneously fission and release neutrons rapidly, for example Californium, Ca252, with a half-life of 2.645 years. During this half-life 3.09 % of the Californium will decay by fission the rest by alpha particle emission. These nuclei can be used as neutron sources in a fission bomb to trigger the nuclear reaction. In a fission bomb polonium 210, Po 210, emits alpha particles into beryllium, Be, which absorbs alphas and emits neutrons.
To create a chain reaction the emitted neutrons must trigger the fission of more than one other uranium nucleus. This can happen if the neutron passes through enough uranium, a so-called critical mass of uranium. The critical mass of U235 is about 50 kg, a 17 cm diameter sphere. The critical mass of Pu239 is 16 kg but by use of a neutron reflector such as tungsten surrounding the plutonium this can be reduced to 10 kg, which is a sphere of plutonium about 10 cm in diameter.
What's Going On?
One question students often ask is, If protons are positively charged and so repel each other, what holds a nucleus together?
The answer is that the strong nuclear force acting between protons and protons, neutrons and neutrons, and protons and neutrons is attractive and stronger than the electrostatic force.
The strong nuclear force between nucleons falls off quickly with distance, it drops off exponentially beyond 1.3 fm. (1 fm = 10^-15 m) The electrostatic repulsion force between protons exceeds the strong nuclear attractive force at distances beyond 2.5 fm. Since the strong nuclear force is so short range it only applies to the nearest neighbor protons and neutrons, however the electrostatic force is long range so in large nuclei the electrostatic force can overcome the nuclear attractive force resulting in alpha particle emission and nuclear fission.
The nuclear bomb is really an electrostatic bomb. The electrostatic force between the protons does the work that is released by the bomb as energy once the strong nuclear attractive force is broken.
Simple models of nuclear fission reactions
Neutron emission model using magnets
Classic experiment with ping pong balls and mousetraps.
References
A good resource to learn more about Nuclear Fission is the Wikipedia
http://en.wikipedia.org/wiki/Nuclear_fission
http://en.wikipedia.org/wiki/Nuclear_weapon_design
Etc.
Joseph Stalin was once handed the "pit" of a plutonium bomb. The plutonium sphere was coated with nickel to block the emission of alpha particles so it could be safely handled. He asked the scientists how he could tell it was really plutonium and not some fake piece of metal. The scientists pointed out that the sphere was warm and would remain warm for thousands of years as the plutonium 239 slowly decayed into lead.
|
Scientific Explorations with Paul Doherty |
|
27 July 2005 |