
New Explorations from the Exploratorium Teacher Institute
by
Paul Doherty and Don Rathjen
Paul's explorations are available on his website at: www.exo.net/~pauld
The Exploratorium Website is at: www.exploratorium.edu
I recently developed explorations to go along with
the Mars Exploration Rover missions. The entire series of
explorations is on line at: Mars
http://www.exo.net/~pauld/lectures/italy/marslectureshort.htm
The following Explorations can be found on my website.


If you create an Atwoods machine with one-half a baseball on one side and a full baseball on the other then the full baseball will accelerate downward at 1/3 g which is close to the 0.38g acceleration of a baseball falling on Mars. Due to the difficulty of obtaining measles springs and inertia free pulleys you will have to add some mass to the full baseball to achieve 1/3g.
Atmosphere Bar


Earth Atmosphere Bar, 1.4 meter long steel bar, Mars atmosphere bar
1.2 cm high steel "bar."
You can order a steel bar 1 inch square and 6 feet
long from a steel supply firm. Cut it to 1.4 meters long The bar will
weigh 14.7 pounds. Put it on you hand and feel one extra atmosphere
of pressure. If you want the metric version hot melt glue a 1
centimeter cube to a full 1 Liter water bottle.
1 kg weighs 10 N and 10 N/cm^2 = 10^5 n?m^2 = 100 kilopascals, about
1 atmosphere
The Venus Bar is a bit of a problem, the atmospheric pressure on Venus is 90 atmospheres and would require a steel bar 130 meters high, the height of a 30 story building.
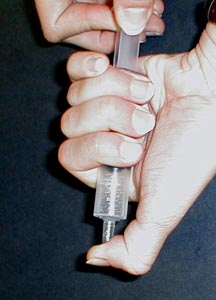
Fill a 10 cc or larger syringe 1/3 full of
water. Close the open end with a finger and pull on the plunger to
reduce the pressure inside the syringe. Let the plunger snap back to
create seed bubbles. Then pull again. Observe the generation of
bubbles inside the syringe. The water is boiling at room temperature
due to the reduced pressure in the syringe. At Martian surface
pressures of 6 millibars water will boil at 0 °C, Mar's surface
is at the triple point of water.
Martian atmosphere CO2 combustion
Get a plastic tank, like a fishtank, place dry ice in the bottom. Put your nose and tongue briefly into the tank to smell and taste Mars. Mars has an atmosphere which is 95% carbon dioxide.
Lower a candle into the tank, notice that it goes
out. Candles need oxygen to burn.
Lower a butane lighter into the tank, the flame leaves the top of the
lighter and burns when it reaches oxygen in the air.

Get two blocks of dry ice from Albertsons or Safeway. Hollow out a tablespoon size hole in one block. Use a fumehood or go outdoors and place the dry ice away from anything flammable. Fill the hole with powdered magnesium. Place a solid magnesium ribbon into the magnesium. Light the ribbon with a propane torch. When the magnesium starts to burn cover it with another block of dry ice. Magnesium will burn in a pure CO2 atmosphere. Never put out a metal fire with a CO2 fire extinguisher.

Shredded nylon ribbon can be charged by rubbing it with wool and then "flown" above a PVC rod which has also been negatively charged by rubbing it with wool. The nylon ribbon is used in oriental markets to wrap packages.
You can also fly a loop tied out of aluminized mylar tinsel (the tinsel is not rubbed first but charge jumps to it as it falls toward the strongly charged PVC.
Recently we discovered that you can charge rectangles smaller than your hand cut from plastic supermarket bags by rubbing them with wool or paper towel. These will then fly over a PVC rod rubbed with wool or paper towel.
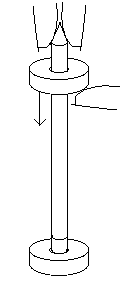
Two donut magnets can be placed on a pencil. Cut off the eraser flush with the metal band, screw a #8 pan head wood screw into the center of the remains of the eraser. One magnet will stick to the steel of the screw. Pull the other magnet up the pencil and drop it. There will be a visual illusion in which the falling magnet seems to pass through the stationary magnet at the bottom of the pencil. This is an elastic collision in which two equal mass objects collide. The moving magnet stops and the stationary magnet pops off at the same speed as the falling magnet.
|
Scientific Explorations with Paul Doherty |
|
25 May 2004 |